In an era of breakthrough advancements in healthcare, it is hard to believe that 90% of blockbuster medicines are only effective for 30-50% of patients. The reason? A “one-size-fits-all” approach to pharmaceutical development and commercialization.
At this year’s 22nd Annual Drug Delivery Partnership meeting, a new approach to the pharma value chain is in discussion. Speaker Barry Frankel explores the power of precision medicine for pharma in his talk, Enabling Drug Development: Artificial Intelligence, Phenotypes and Genomics Integrated for Precision Medicine. Precision medicine aims to personalize healthcare, factoring in individuals’ traits—genetics, lifestyle, etc.—to develop targeted approaches to diagnosis, treatment, and prevention for patients.

The incorporation of genomic information into pharmaceutical development is on the rise; Frankel highlights that genomics adoption is now faster than Moore’s Law*. However, the industry is still facing barriers to full-on adoption. Drugs based on genetic targets have a success rate 1.5-2X better than their counterparts that don’t include genetic information, but identifying the genetic variant causing a disease is like trying to find a needle in a haystack.
In steps FDNA and their deep learning technology. The technology captures physiological information, biometric data—such as facial photos—and clinical notes, curated in a proprietary database, to identify disease-causing genetic variants with higher accuracy in a process called “next-generation phenotyping” (NGP).
NGP is the use of computational techniques to integrate phenotypic data into the analysis of human health, including the capturing, structuring, and interpretation of complex clinical information. The correlations found between phenotypic data and genomic data improve and accelerate targeted diagnostics, therapeutics development, and patient care.
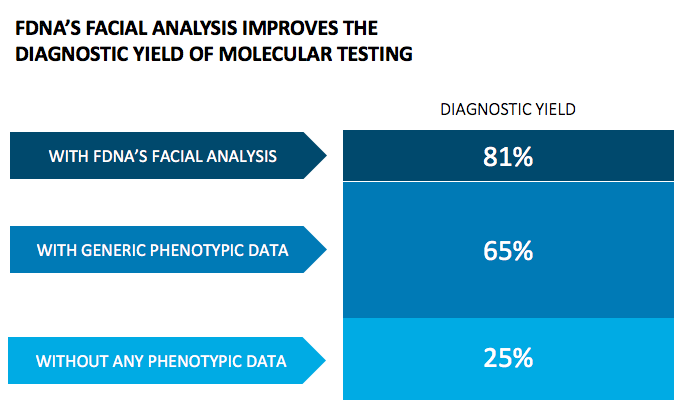
“We estimate that the addition of phenotypic features increases the diagnostic yield to about 60% (from 25% without). When adding facial analysis, FDNA’s technology, to that process, the diagnostic yield increases to more than 85%.” Dr. Peter Krawitz University Hospital Bonn, Germany, Principal Investigator of PEDIA
NGP has the capacity to complement NGS across the entire Pharma chain, from discovery to development to commercialization. NGP helps researchers identify and target disease biomarkers, enable earlier and more targeted patient diagnosis for trial recruitment, and personalize therapeutic approaches.
Interested in learning more about using NGP for the benefit of your development and commercialization activities? Learn more at www.FDNA.com
*Source: National Institute of Health, National Human Genome Research Institute (7/17), Biology Reference, Illumina